PROCESSING
By Stephanie Nguyen, Nancy Dobmeier, CQA, CHA, and David Albrecht
Justification of Run Time: How to Determine and How to Extend
Understanding what impacts run time is essential for proper sanitation

Photo credit: MediaProduction/E+ via Getty Images
SCROLL
DOWN
How long do your production lines run before executing a sanitation event? Hours? Days? Weeks? Do you have the necessary documentation to justify the run times? As a common practice, many facilities conduct routine daily sanitation—typically a third-shift operation. However, regulations do not require that sanitation must occur within 24 hours¹, nor is there a free pass² for running up to 24 hours without a cleaning measure (or assessment).
While some may argue that with the U.S. Department of Agriculture (USDA)’s Less than Daily Sanitation guidance published in 2009, it is implied that a line may run for a full day before it is cleaned. However, the guidance says³ that “there have never been FSIS [Food Safety and Inspection Service] regulations that required an establishment to conduct cleanup every twenty-four hours or within any other specified period.” What the regulations do require is that sanitation must be done as necessary/appropriate, and the rationale should be captured in the supporting documentation for the facility’s food safety/Hazard Analysis and Critical Control Points plan.
The Code of Federal Regulations section pertaining to USDA-FSIS establishments (Title 9, Chapter III, Subchapter E Part §416.4 Sanitary Operations) directs that all food contact and nonfood contact surfaces (e.g., surfaces of utensils, equipment, and the facility) must be cleaned and sanitized as frequently as necessary to prevent the creation of insanitary conditions and the adulteration of product. U.S. Food and Drug Administration regulations in 21 C.F.R. [Part 117, Subpart B §117.80 Processes and controls, Manufacturing operations section (c)(2)] state that “all food manufacturing, processing, packing, and holding must be conducted under such conditions and controls as are necessary to minimize the potential for the growth of microorganisms, allergen cross-contact, contamination of food, and deterioration of food.” Sanitation controls are one of the preventive controls intended to help control such conditions and must be implemented as appropriate to the facility and the food…to control those hazards [Subpart C §117.135 (c)(3)].
Determining frequency of sanitation is not arbitrary and requires collection of data and documentation. Using “that’s the way we have always done it (and no one has gotten sick)” does not stand as reasonable rationale. To justify how long a line or a facility can run between full chemical cleans or interdictive cleaning measures (e.g., rinse down, scrape down), a science-based assessment must be conducted to prove that sanitary conditions are maintained throughout a run. This article will outline parameters that should be considered in the assessment to justify length of production runs. These parameters will depend on the type of product manufactured [e.g., ready-to-eat (RTE)/not RTE or low-/high-moisture foods] and may not apply to all situations.
Parameters Used to Justify Production Run Length
Location of data collection. The analysis and microorganisms studied when performing an assessment will differ, depending on where in the process the data are collected—including areas before and after a lethality step, if applicable. Upstream of a lethality step, the primary focus may be on high-moisture matrices and controlling run times so that the growth level of pathogens does not approach the point of toxin production or exceed the capability of the thermal process to eliminate or reduce vegetative pathogens to acceptable levels. Downstream of a thermal process, the focus may shift to indicator testing on food contact surfaces and environmental pathogen swabbing. Throughout the process, validation of sanitation practices needs to be conducted to ensure acceptable cleaning of equipment is executed.
High-moisture matrices. At any point in the process, including before a lethality step or in a work-in-process (WIP) material, when product is in a high-moisture form (having a water activity > 0.85), a risk assessment needs to be conducted and mitigation strategies need to be implemented to prevent the levels of toxigenic pathogens such as Staphylococcus aureus and Bacillus cereus from growing to critical levels and producing heat-stable toxins. Equipment contact times for these materials (thus potentially driving overall production run length or time between cleanings) need to be specific to the materials themselves. The data collected for the risk assessment should include attributes of the matrix, recorded temperatures, and product buildup areas on the equipment. For additional guidance on assessing a high-moisture matrix, see a previous article⁴ in Food Safety Magazine.
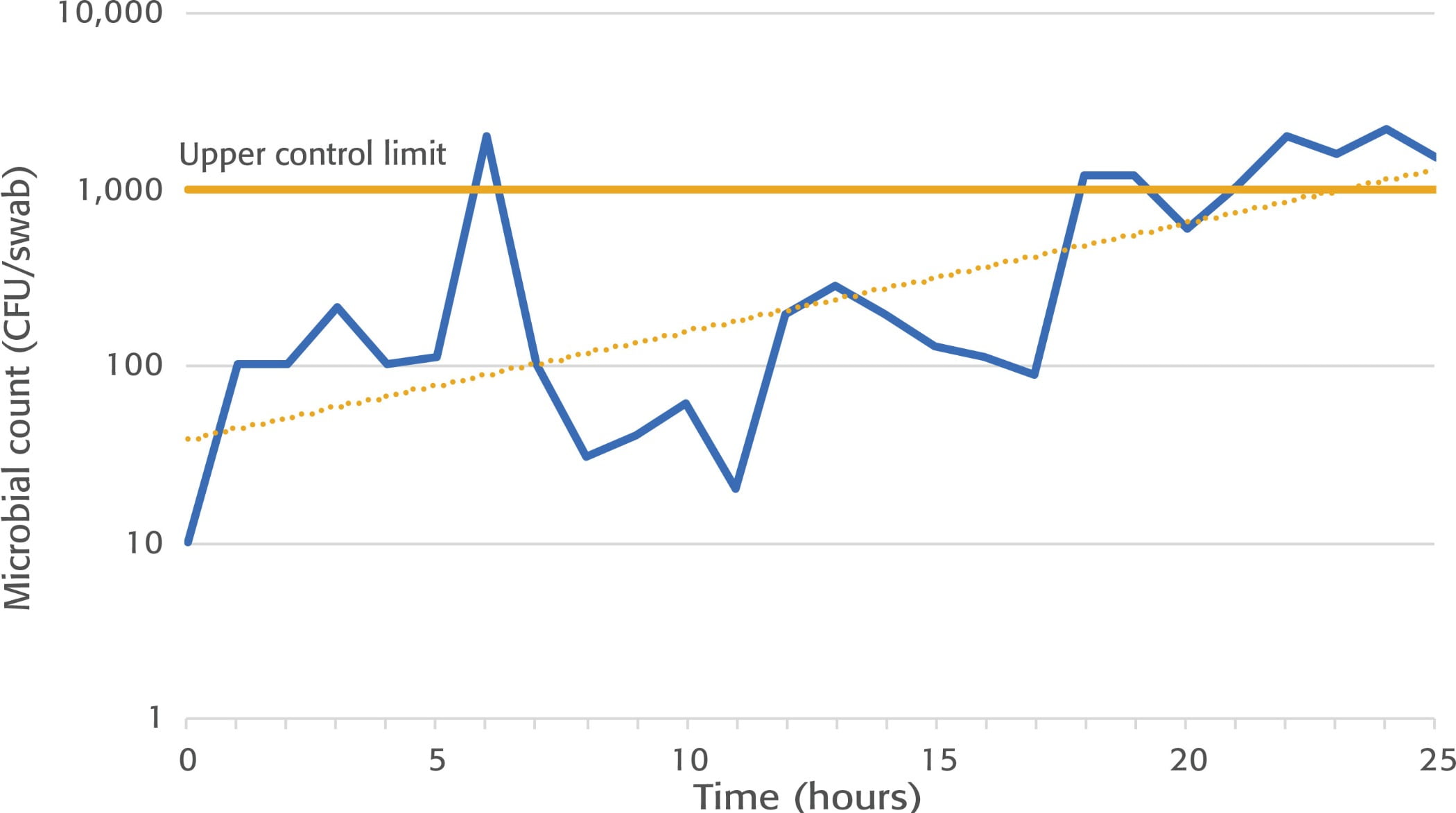
Food contact surfaces in a primary pathogen control area. If your process includes a validated lethality step, or production of a ready-to-eat product, food contact surface sampling (swabbing) in areas after the kill step and up to primary packaging may help determine whether insanitary conditions are developing. Typically, an aerobic plate count is conducted on the swabs. Other indicators such as Enterobacteriaceae, coliforms, Listeria-like organisms, and yeasts and molds (particularly for surfaces conveying low-moisture foods) can be a good indication⁵ of the hygienic and sanitary state of a surface. Determine the specific food contact areas you will swab and continue to swab those general areas throughout the study. A 4-by-4-inch area (or around 16 square inches) is often used. Do not swab the exact same spot at each swabbing event, because it could give you artificially lowered results. Swab an adjacent area of equal size, as the previous swabbing has essentially cleaned the site. A baseline must be established before conducting a length-of-run study. To do so, collect swabs at the end of a typical run before sanitation. If your goal is to establish the scientific rationale for your current run time, compare these data with swabs collected right before start-up. When doing a study to extend the length of a previously validated run time, collect swabs at the end of the normal run hours and at the end of the added time, again from the same general/adjacent areas. In the United States, there is currently no agreement on limits⁶ for indicator bacteria to determine whether a process is in control. Regardless, look for trends in the data and utilize statistical process control (SPC) methodology to evaluate whether run time alters the microbial results on food contact surfaces. Figure 1 demonstrates an example situation where a manufacturer may want to discontinue production around 18–21 hours and conduct a sanitation event, as multiple data points are trending above the upper control limit. SPC may help detect outliers, and if the count trend continues upward, this may be a clear indication that the process is no longer in control.
FIGURE 1. Example of an SPC Chart
Environmental monitoring for pathogens. As a run progresses, the movement of people and materials throughout the facility increases. This, coupled with less frequent cleanings, may increase the risk for pathogen positives (typically Salmonella spp. and Listeria monocytogenes and/or Listeria spp.) in the environment. To support a run-time extension, collect zone 2 and zone 3 swabs after a full clean immediately before start-up to verify the negative pathogen status of areas. Then, collect swabs of the same areas at a set frequency (e.g., every 8 hours). The comparison of these “before,” “during,” and “end” data, along with historical data, will help identify whether extending the length of time between cleanings increases the risk of getting positive swab results in the general environment. If results are unfavorable, follow your facility’s corrective action requirements, including any escalation practices. For operations involving low-moisture foods, environmental pathogen testing is probably more important than zone 1 swabbing for indicators. If extending the length of time between sanitation events increases the percentage of positives in the environment when compared with established production times, reevaluation of sanitation controls (including hygienic zoning practices) may be necessary.
Finished product testing. Foundational food safety programs including Good Manufacturing Practices, verification of temperature controls, and hygienic zoning must be strictly adhered to when conducting a study for the justification of run time. Additionally, when finished product testing for pathogens is conducted, results can aid in the disposition of product manufactured during the study. Consider the logistics of finished product testing—lot separation, a robust sampling plan, and holding of product—as these need to be implemented during testing. Keep in mind, finished product testing on its own is not always a good indicator of whether a process is in or out of control (e.g., time/temperature controls of WIP). Even though vegetative cells are eliminated during the thermal process, heat-stable toxins may have already been formed in the WIP from such organisms as S. aureus and B. cereus if appropriate mitigation strategies are not in place. Typical finished product testing of vegetative cells will not detect this condition.
Sanitation considerations. As production time increases, the food product soils that build up on equipment surfaces may need to be addressed more intensely. Buildup can become thicker and hardened, which may change the methods used to normally remove it during the end-of-run sanitation event. Increased chemical concentrations could be needed; water flow, pressure, and temperature also may need to be adjusted. Labor costs should be factored into the cost of extending a production run. Determine whether you can manage interdictive cleaning needs during operations with your current staffing, or if adding labor reduces the savings from extending the run. A run-time extension may push the sanitation cycle to a different time in the day. Moving away from a set cleaning time to one that pushes sanitation into other shifts or even multiple shifts can create challenges in managing the process efficiently and effectively and may increase the sanitation cleaning turn time. Therefore, cost in the form of increased chemicals, utilities, and labor may be a necessary consideration if an extended production run is desired. Effectiveness of end-of-run sanitation must be supported by scientific data—that is, validating with microbiological testing that sanitation is still effective on the additional soils that may accumulate given a longer run time. Another consideration is the ability to execute the master sanitation schedule (MSS) for cleaning overheads or peripheral support areas in a timely manner. Extending the run time may result in shortened windows of opportunity to conduct these activities. Deviations from the MSS could contribute to an upward trend in environmental pathogens in the facility.
“Determining frequency of sanitation is not arbitrary and requires collection of data and documentation.”


Product quality and employee safety. Assuming all food safety goals are met during the study of extending your run time, sacrificing quality of product for longer run periods is another consideration. During the study, product should also be evaluated to ensure that quality is not adversely affected. For example, consider a product that has a seasoning topically applied. Seasoning could build up over time and break off into the finished-material stream, causing an undesirable experience for the consumer. This can be prevented by limiting the run length or inserting an interdictive scrape down. Equally important to food safety is the safety of employees in the facility. For example, in a frying operation, soils in the form of oil or crumbs on the floor may accumulate and create a slip hazard to employees if not cleaned at a specific frequency. In both situations, factors other than food safety may be limiters in production length.
Other considerations when doing a study to extend run time. If the intent is to increase the run-time length, consider the logistics around extending the run time and implications for product disposition. It may seem easiest to jump straight from the current production time to the desired run length (24 to 72 hours, for example). However, it is a balance between getting to your end goal and how much product your operation is willing to risk. If unfavorable results from sanitation, environmental monitoring, finished product, and/or product quality testing are obtained, there is potential risk that all product made between the current run time and the extended time is compromised and must be disposed of appropriately. This can be a very costly exercise for the company. Conversely, if a stepped approach is taken (e.g., ramp up from 24 hours to 72 hours in 8-hour increments), it may take longer to achieve the final results, but less product (8 hours’ worth in this example) is put at risk. An additional upside is that the collected data should clearly demonstrate when the parameters start to fail. The important thing to note is that running under control as supported by all available data is increasingly important to food safety as well as the financial health of the organization as run times are extended. Thus, the decision to extend run times should not be taken lightly.
Run-time determination, whether collecting data to support the length of a run for a current process or a future process, or to extend a process, must be conducted methodically. Without a science-based rationale for maximum production times, food safety, employee safety, facility finances, and quality could be at risk. There are several considerations ranging from environmental monitoring to modification of sanitation programs that need to be addressed as part of the justification. By conducting a thorough assessment, food manufacturers will have taken a necessary step toward meeting regulatory expectations and producing microbiologically safe products. As with any program related to the safety of food manufacturing, reach out to a subject-matter expert to accurately design a study that reflects the specifics of your food type and manufacturing facility.
References
1. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=117.35.
3. www.fsis.usda.gov/wps/wcm/connect/2398294b-9e25-4382-bd85-768dd52d3aac/5000.5Rev1.pdf?MOD=AJPERES.
4. www.food-safety.com/articles/6055-time-flies-when-temperatures-rise.
5. www.food-safety.com/articles/3850-indicator-organism-assays-chaos-confusion-and-criteria.
6. www.fsis.usda.gov/wps/wcm/connect/7fb1a71f-27fa-4b04-a065-82f65e21cb03/NACMCF-Process-Control-111714.pdf?MOD=AJPERES.
Stephanie Nguyen is working as a principal microbiologist, Food Safety and Microbiology, at Conagra Brands. She also works in the area of thermal processing and is a recognized Process Authority.
Nancy Dobmeier, CQA, CHA, is working as a principal microbiologist, Food Safety and Microbiology, at Conagra Brands and has been working in microbiology/food safety for 30 years.
David Albrecht is working as a manager of sanitation at Conagra Brands, having over 20 years of quality and sanitation experience in the areas of frozen food, snacks, canning, and processed meat products.

